Image courtesy of Alachua County via Public Domain
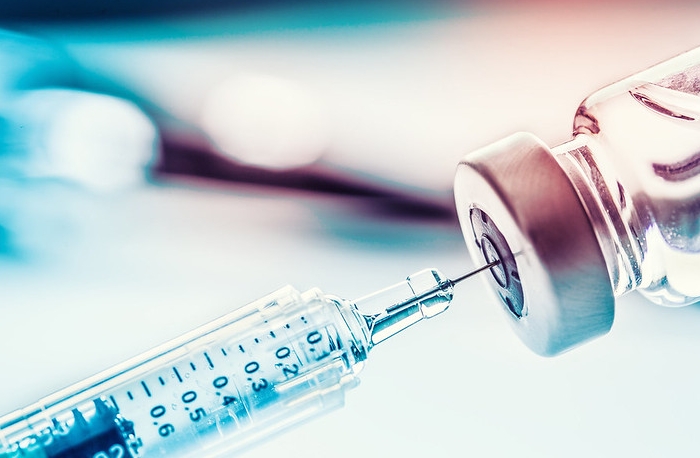
One year after the World Health Organization (WHO) declared the novel coronavirus (COVID-19) outbreak a pandemic [1], several COVID-19 vaccines were developed and received emergency use authorizations to be administered globally. One such vaccine was developed by AstraZeneca and the University of Oxford, which was authorized for emergency use by WHO’s Emergency Use Listing (EUL) in February 2021. The EUL assesses science and safety factors, such as quality and efficacy, of COVID-19 vaccines [2]. The addition of another COVID-19 vaccine to the arsenal is positive news as it provides more doses available to the public. Additionally, the company reports an efficacy of 79% in the prevention of symptomatic disease and 100% efficacy in preventing severe disease [3], which are similar to the Pfizer and Moderna vaccine efficacy rates already approved for emergency use in the United States (efficacy of 95% and 94.1% at preventing symptomatic disease, respectively) [4].
However, since the deployment of the AstraZeneca/Oxford vaccine in the European Union, there are reports of rare blood clots presenting in people who recently received the AstraZeneca/Oxford vaccine. After these reports, thirteen European countries suspended administration of this vaccine [5], and others stopped administering specific batches of the AstraZeneca/Oxford vaccine [6]. The European Medicines Agency’s (EMA) safety committee investigated the reports and declared that, “the vaccine is not associated with an increase in the overall risk of blood clots (thromboembolic events) in those who receive it; however, the vaccine may be associated with very rare cases of blood clots associated with thrombocytopenia…” [6]. Furthermore, on 18 March 2021, EMA reported the benefits of receiving the AstraZeneca/Oxford vaccine still outweigh risks, arguing there is no increased risk of blood clots, citing the overall number of reported thromboembolic events after vaccination are lower than what one would expect in the general population [6]. However, the safety committee will continue to monitor the reports and will undertake additional reviews of the risks [6].
After EMA’s call to continue use of the AstraZeneca/Oxford vaccine, Germany, France, Italy, and Spain resumed administration of this vaccine. Others continue to halt administration of the vaccine until further review by their own government’s public health institutions [7,8].
Citing the vaccine’s efficacy, AstraZeneca/Oxford announced plans to apply to U.S. Food and Drug Administration (FDA) for Emergency Use Authorization on 22 March 2021[9]. Furthermore, the White House declared its trust in the vaccine and announced that, if approved, the vaccine will be integrated into the vaccine distribution system in the United States [10].
During times like this, it is important to trust our scientists and public health professionals and to follow the guidelines they set forth. If the FDA approves the AstraZeneca/Oxford COVID-19 vaccine for emergency use, we should not hesitate to receive it if it is offered.
Update, 23 March 2021:
Less than 24 hours after AstraZeneca announced the vaccine’s efficacy, the Data and Safety Monitoring Board (DSMB) notified the U.S. National Institute of Allergy and Infectious Disease (NIAID), the Biomedical Advanced Research and Development Authority (BARDA), and AstraZeneca that there is concern outdated information from the vaccine clinical trial was included, which, therefore, may provide an incomplete picture of the efficacy data [11].
AstraZeneca responded stating the efficacy data published on 22 March 2021 were based on a “pre-specified interim analysis with a data cut-off of 17 February” [12]. The company is completing the validation of the primary analysis and will work with the DSMB to share the most up to date data [12].
This story is ongoing. Updates will be posted as information becomes available.
Update, 25 March 2021:
Early this morning, AstraZeneca published the results from the primary analysis of the Phase III trial in the United States. They announced the vaccine has 76% efficacy against symptomatic COVID-19 and 100% efficacy against severe disease or hospitalization [13]. These results do not differ much from the pre-specified interim analysis results the company announced on 22 March. Additionally, they state that there we no safety concerns identified in the Phase III trial.
AstraZeneca still plans to submit for Emergency Use Authorization with the FDA and will also submit the primary analysis for peer-review in the coming weeks [13].
References:
[1] https://pubmed.ncbi.nlm.nih.gov/32191675/#:~:text=Abstract,a%20global%20pandemic%20(1)
[5] https://www.bbc.com/news/world-europe-56440139
[7] http://outbreaknewstoday.com/finland-reports-two-cvst-cases-suspends-astrazeneca-vaccine-use-86093/
[11] https://www.nih.gov/news-events/news-releases/niaid-statement-astrazeneca-vaccine